Introduction:
Waldenström's macroglobulinemia is a rare low-grade lymphoplasmacytic lymphoma characterized by CD20 expression on malignant cells and produces a monoclonal immunoglobulin M (IgM). Rituximab is a chimeric monoclonal antibody against CD20 antigen and the most widely used therapeutic agents in WM. Rituximab works by adhering to CD20, causes B-cell lysis mainly through antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. Cyclophosphamide is an alkylating agent binds to DNA. Its cytotoxic effect is mainly due to cross-linking of strands of DNA and RNA, and to inhibition of protein synthesis. Our objective is to analyze and summarize the published literature for the efficacy of regimens containing both rituximab and cyclophosphamide for the treatment of Waldenström's macroglobulinemia (WM).
Methods:We performed a comprehensive literature search on articles following PRISMA guidelines. Beginning with articles published after January 2005, we used databases like PubMed, Embase, Clinicaltrials.gov, Cochrane Library, and Web of Science. A total of 585 articles were identified initially, and after a detailed screening, we finalized 10 studies involving 425 WM patients.
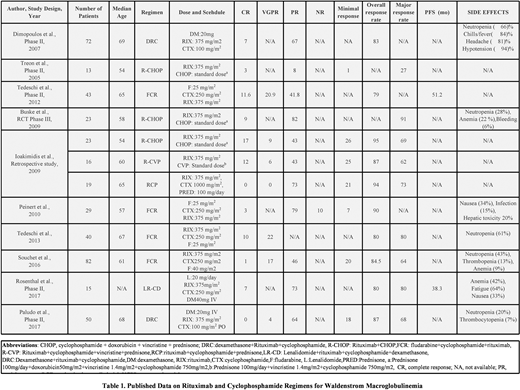
Results:The total number of patients were 425. The dose was 375 mg/m2. The complete response (CR) ranged from 3-19%, very good partial response (VGPR) ranged from 4-22%, and the partial response (PR) ranged from 8-82%. The overall response rate (ORR) ranged from 79-95%.
Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone (R-CHOP):
In a retrospective study by Ioakimidis et al., included 23 WM patients were given R-CHOP. The ORR was 95%, with 69% achieving a major response, 43%, PR, and VGPR OF 9%. In a randomized control trial by Buske et al., N=23, the major response rate was 91%, and PR was 82%. Treon et al. (N=13) observed a major response rate of 27%. (Table 1).
Fludarabine, Cyclophosphamide and Rituximab (FCR):
In a retrospective study by Peinert et al., involving 29 patients receiving the FCR regimen, the ORR was 90%, with 79%, 3%, and 10% of patients achieving PR, CR, and NR, respectively. In a study by Tedeschi et al., involving 40 patients, ORR was 80%, and the major response rate was 80%. Souchet et al., N=82 reported an ORR of 84% and a PR of 46%. Progression-free survival (PFS) was 51.2 months for Tedeschi et al.
Dexamethasone, Rituximab and Cyclophosphamide (DRC)
In study Paludo et al., (N=50), receiving the DRC regimen, the highest ORR was 87%, PR 64%, and major response was 68%. In a phase II study by Dimopoulos et al., (N=72), the ORR of 82% and PR of 67% was observed. PFS was 24 months in this study. (Table 1)
4.Rituximab, Cyclophosphamide, Vincristine, and Prednisone (R-CVP)
In study Ioakimidis et al, N=16, the ORR was 87%, the major response rate was 62%, and the PR was 43%.
Rituximab, Cyclophosphamide, and Prednisone (RCP) Ioakimidis et al., reported 19 patients who were given RCP. The ORR, PR, and the major response rate was 94%, 73%, and 73%, respectively.
Lenalidomide, Rituximab, Cyclophosphamide, and Dexamethasone (LR-CD)
A study by Rosenthal et al., 15 patients were given LR-CD. ORR was 80%, with a major response rate in 80% and PR in 73% of patients. PFS was 38 months.
Conclusion:
Rituximab and cyclophosphamide, in combination regimens for the treatment of WM showed the overall response rate of 95%. Neutropenia was the dominant side effect reported in these regimens. There is a paucity of phase 3 randomized trials demonstrating a clinical benefit of anyone regimen over another. We recommend future randomized prospective trials better to understand the efficacy and safety profile of regimens containing both rituximab and cyclophosphamide base combinations.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal